|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
 |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
 |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
2011, Vol. 6 No. 2, Article 95
In Vitro Sensitivity of Aspergillus Species Isolated Christudas Silvanose*, Tom Bailey and Antonio Di Somma
Dubai Falcon Hospital,
*Corresponding Author; e-mail address: [email protected]
ABSTRACT One hundred seventeen fungal isolates , A. Fumigates (71), A. Flavus (23), A. Niger (14), and A. Terreus (9) obtained from the air sac of falcons affected with respiratory Aspergillosis, were subjected to sensitivity test to determine in-vitro sensitivity of different Aspergilus species against widely used anti fungal agents. All the (117) isolates were found sensitive to voriconazole and posaconazole and ninety seven percent (114) of the isolates were sensitive to itraconazole with a MIC ≤1µg/ml. Eighty percent (94) of the isolates, including 88.7% (63) of A. fumigatus, 42.8% (6) of A. niger, 100% (16) of A. flavus and 100% (9) of A. terreus were resistant to amphotericin B with a MIC >1 µg/ml. Eighty six percent (100) of the isolates, including 87.3% (62) of A. fumigatus, 74% (17) of A. flavus, 86% (12) of A. niger and 100% (9) of A. terreus were resistant to ketaconazole with a MIC >1µg/ml. All isolates were resistant to 5-flucystosine with a MIC ≥ 2µg/ml; caspofungin with a MIC ≥ 16µg/ml and fluconazole with a MIC ≥ 256µg/ml. KEY WORDS Aspergillus, Amphotericin B, Itraconazole, Fluconazole, Ketaconazole, Voriconazole, Caspofungin, 5-Flucystosine. INTRODUCTION Aspergillosis caused by Aspergillus fumigatus, Aspergillus flavus and Aspergillus niger is a common avian mycosis that affects captive waterfowl, wading birds, penguins, raptors, ostriches, pheasants and passerines. It causes considerable morbidity and mortality in raptors and falcons trained for falconry. The disease involves respiratory tract mainly, trachea, lungs and air sacs Lumeij et.at. (2000), Redig (2008). Owing to the pathogenic nature of fungi and development of resistance against commonly used fungicides, evaluation of sensitivity pattern of pathogenic fungi has become important in avian medicine Silvanose et.al. (2006). Present investigation was under taken to determine the sensitivity pattern of fungi isolated from the respiratory tract of falcons using the micro-dilution method with commercially available dried antifungal panels of Sensititre Yeast One (Trek, USA). MATERIALS AND METHODS
Biopsy samples were collected during endoscopy from the air sacs of one hundred and seventeen falcons (Table-1) affected with fungal infection of the lower respiratory tract. Samples were cultured in Sabouraud’s chloramphenicol agar (SCA) and incubated at 37°C for 3-5 days. Fungi were identified by culture appearance and morphological characteristics under the microscope using lactophenol aniline blue stain preparation.
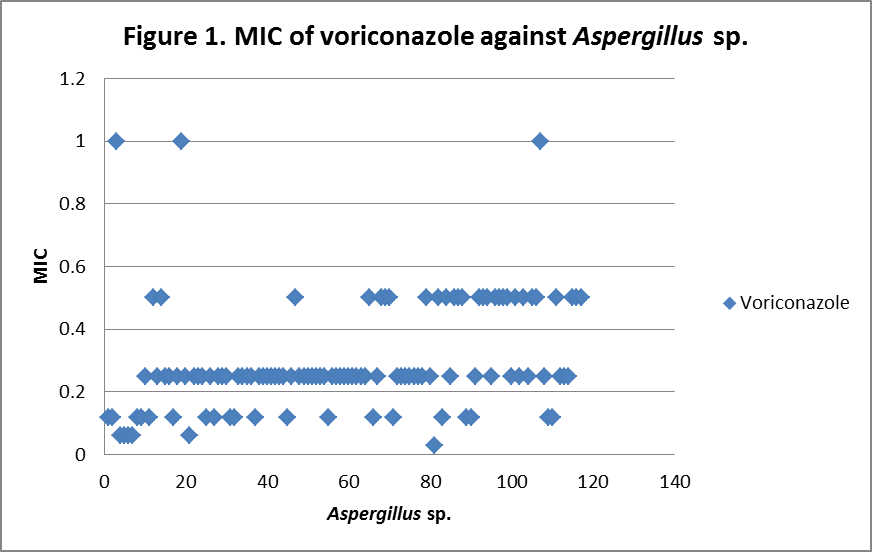
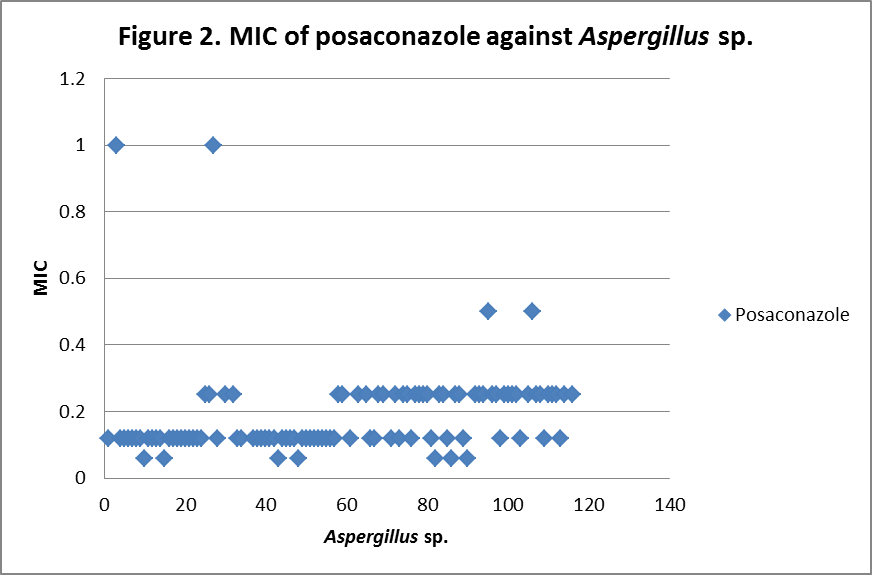
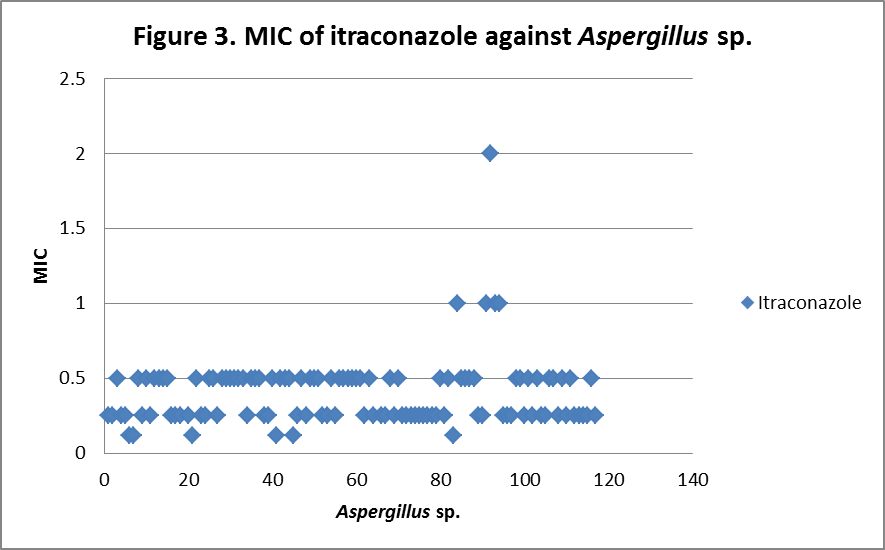
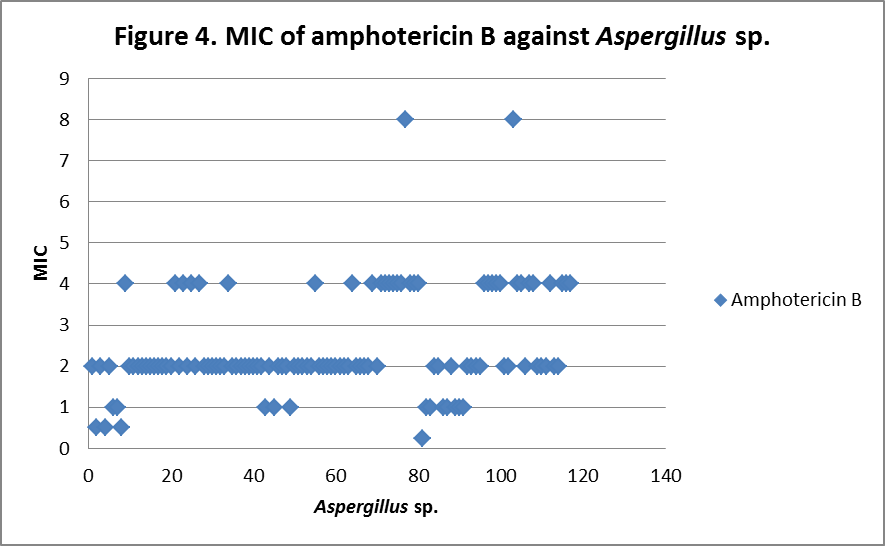
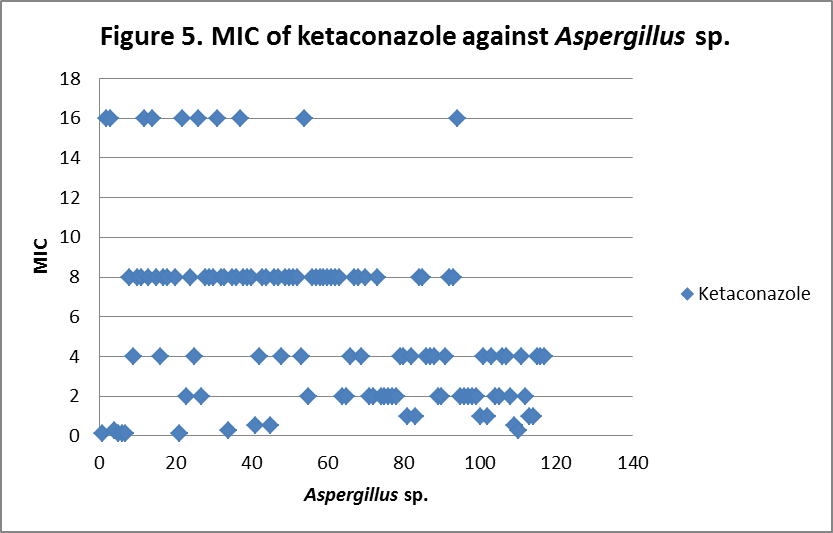
RESULTS The fungi isolated collected from the air sac of falcons include 61 % (71/117) A. fumigatus, 20% (23/117) A. flavus, 12 % (14/117) A. niger, and 8 % (9/117) A. terreus. All the (117) isolates were sensitive to voriconazole and posaconazole with a MIC ≤1µg/ml. 97.4% (114) and 98.3% (115) of isolates were sensitive at a MIC≤0.5 µg/ml of voriconazole and posaconazole respectively. 97.4% (114) of the isolates were sensitive to itraconazole with an MIC ≤ 1µg/ml. Eighty percent (94) of the isolates, including 88.7% (63) of A. fumigatus, 42.8% (6) of A. niger, 100% (16) of A. flavus and 100% (9) of A. terreus were resistant to amphotericin B with a MIC >1 µg/ml. Eighty six percent (100) of the isolates, including 87.3% (62) of A. fumigatus, 74% (17) of A. flavus, 86% (12) of A. niger and 100% (9) of A. terreus were resistant to ketaconazole with an MIC > 1µg/ml. All isolates were resistant to 5-flucystosine with a MIC ≥ 2µg/ml; caspofungin with a MIC ≥ 16µg/ml and fluconazole with a MIC ≥ 256µg/ml. All MIC (µg/ml) results including median and ranges are presented in Table 1. Figures 1 to 5 show detailed MIC patterns of each antifungal with MIC90 and MIC50. The quality control results were within the accepted ranges. DISCUSSION
Posaconazole, voriconazole and itraconazole had the lowest median MIC against all most all the species of Aspergillus. These observations fall in line with reports of Silvanose et.al. (2006), Ken et.al. (2009) and contradict the findings of Susan et.al, (2006), Beernaert. et.al, (2009). In comparison to azoles, itraconazole, voriconazole, and posaconazole showed a similar pattern of MIC90 and MIC50, while ketaconazole showed higher MIC90 and MIC50 and these results were in agreement with the previous reports Pfaller et al, (2000), Beernaert. et.al.(2009), Ken et.al. (2009). During present study, three isolates (2.6%) were found resistant to itraconazole with MICs of 2µg/ml, 8µg/ml and 16µg/ml and two of these showed sensitivity at MIC of 1µg/ml to posaconazole and no cross resistance was observed. Previously, cross resistance between itraconazole and posaconazole was reported in 53.50% Pfaller et.al. (2009), between itraconazole and voriconazole in 74% Susan.et.al. (2006) of isolates among the itraconazole resistant Aspergillus sp. The frequency of itraconazole resistance was documented as 2 - 6 % of clinical isolates of
A. fumigatus and azole cross-resistance had been reported infrequently associated with treatment failure. Susan.et.al. (2007) .The primary mechanism of resistance described for clinical isolates of
A. fumigatus is mutation in the target protein. The cyp51A gene encodes the target of azoles, lanosterol 14
α-dimethylase, and this enzyme catalyzes a step in the biosynthetic pathway of ergosterol which is an essential cell membrane component of filamentous fungi. The mutation reduces the binding of azoles to the enzymatic site and it could result in a resistance mechanism Susan et al, (2006).
REFERENCES
TABLES Table-1: Biopsy samples collected from various Falcon species
Table-2: MIC of antifungals against Aspergillus sp isolated from the air sac of falcons
a - Median b -Range n- Number of isolates FIGURES
MIC90 - 0.33µg/ml, MIC50 - 0.18µg/ml
MIC90 - 0.20µg/ml, MIC50 - 0.18µg/ml
MIC90 - 0.34µg/ml, MIC50 - 0.25µg/ml
MIC90 – 2.75µg/ml, MIC50 - 2µg/ml
MIC90 - 4µg/ml, MIC50 - 2.37µg/ml
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Copyright © Vet Scan 2005- All Right Reserved with
VetScan |
Home | e-Learning |Resources | Alumni | Forum | Picture blog | Disclaimer |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
powered by eMedia Services |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||