|

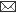
2011, Vol. 6 No. 1, Article 85
Postpartum Ovarian Cyclicity of Zebu Cows in Bangladesh
Taslima Akter*1, M. Mostofa Kamal2, Anup Kumar Talukder3, Zesmin Akter1,
Farida Yeasmin Bari1 and Mohammed Shamsuddin1
1Department of Surgery and Obstetrics, Faculty of Veterinary Science,
Bangladesh Agricultural University, Mymensingh 2202, Bangladesh
2Department of Livestock Services, Dhaka, Bangladesh
3Department of Medicine and Surgery, Faculty of Animal Science and Veterinary Medicine,
Patuakhali Science and Technology University, Barisal 8602, Bangladesh
*Corresponding Author;
e-mail address: [email protected]
ABSTRACT
Postpartum luteal function and oestrus were studied using radioimmunoassay (RIA) of milk progesterone in relation to body weight, body condition score (BCS) and milk production. Randomly fifty cows were included from selected areas of Mymensingh District of Bangladesh. In 52% of the cows (n=26) ovarian cyclicity resumed by Day 80 postpartum. Thirty two cows (64%) showed their ovarian cyclicity by Day 120 postpartum. Cows with low body weight (219.2±0.3 kg) at calving required more days (234.1±66.6 days) to initiate postpartum ovarian cyclicity (107.0±5.0 days) than those of higher body weight (286.6±89.0 kg) (p<0.05). Cows with higher BCS at calving (3.0±0.9) and at ovulation (2.7±0.9) had calving to ovulation interval 107.0±5.0 days. Cows with lower BCS at calving (2.5±0.6) and at ovulation (1.9±0.3) required long time (234.1±66.6 days) (p<0.05). Cows with higher milk production (6.3±3.5 kg/L) took shorter (77.4±6.5 days) calving to ovulation interval than that (234.1±66.6 days) of lower milk production cows (1.8±0.9 kg/L) (p<0.05). It can be safely inferred that that by improving the body condition score and body weight at postpartum period, earlier ovarian cyclicity can be achieved.
KEY
WORDS
Body weight, BCS, milk yield, ovarian cyclicity.
INTRODUCTION
To achieve satisfactory economic benefit from the dairy industry, inter-calving interval should not exceed 365 days (Haresign et al., 1983; Opsomer et al., 1996; Shamsuddin et al., 2006). To get calf at every 365 days, the average interval between calving and onset of ovarian rebound should not exceed 60 days (Morrow, 1986). Prolong interval (>85 days) between calving and onset of ovarian function is regarded as one of the most important gynecological problems responsible for failure to maintain optimum reproductive efficiency which in turn causes economic loss to the dairy farmer (Coleman et al., 1985; Shamsuddin et al., 2006; Kamal, 2010). Usually, Restoration of postpartum ovarian function in Bos taurus cows occurs in between 18 and 30 days after parturition, provided they are not having had any periparturient disorders (Torres, 1997). But the intervals between calving to first estrus in non-descript local zebu cows often exceeds 120 days (Shamsuddin et al., 2006) which certainly causes great economic loss to the dairy farmers.
Among various factors, nutrition, body weight, body condition score and milk yield are important determinants of the animal to the initiation of ovarian cyclicity (Garcia, 1988; Fitzpatrick et al., 1994). Monitoring progesterone concentrations in body fluid (milk and plasma) by radioimmunoassay (RIA) technique indicate the functional status of the ovaries in cyclic, pregnant and non-cyclic uterine diseased animals (Claycomb et al., 1996). The aims of the present study were:
1) to determine the onset of ovarian cyclicity through measuring progesterone concentration in milk by radioimmunoassay
2) to evaluate the effects of body weight, condition score and milk yield on the onset of postpartum ovarian cyclicity
MATERIALS AND METHODS
Animal Management
The present investigation was conducted at selected areas of Mymensingh District of Bangladesh. Fifty lactating cows from smallholder farms were registered within 1 week after calving with relevant information of the farm and cattle recorded in respect of body weight, body condition score, milk production and the occurrence of any post-parturient disorders. Routine deworming against liver flukes, round worms was in practice and the cows were vaccinated routinely against anthrax, black quarter, hemorrhagic septicemia and foot and mouth diseases. The cows were stall fed supplied with approximately 5 kg straw, 10-15 kg green grasses and 3-5 kg concentrates per cow per day in 2 splits.
Determination of body weight
The body weight of the cows was measured and recorded at an interval of 10 days beginning immediately after calving up to 120 days postpartum, using a standardized tape obtained from the Swedish Association of Livestock Breeding and Production, Eskilstuna, Sweden (Comb MAAI).
Determination of condition score
The nutritional state of individual cow was determined by scoring body condition using 1-5 scales (0.5 fraction between 2 scores) on the basis of bony prominence and deposition of subcutaneous fat as described by Nicholson and Butterworth’s (1986). The lowest score was given to the thin cows whereas the highest score was given to the fat one (Shamsuddin et al., 1997).
Collection of milk samples
Milk samples were collected at a 10-day’s interval from day 10 postpartum to exhibition of the first postpartum oestrus. Two more samples were collected at 10 days interval, after the occurrence of oestrus. Sodium azide tablet (Mark, Germany) was used as preservative (8mg/10ml milk). Milk samples were centrifuged at 2000g for 15 minutes and defatted milk was separated from the supernatant fat by using a Pasteur pipette and stored at -200C until analyzed.
Determination of progesterone concentrations
The progesterone concentration in the defatted milk was determined by using a solid phase radioimmunoassay (RIA) technique. The RIA kits for milk progesterone were supplied by laboratory of the International Atomic Energy Agency (IAEA), Vienna, Austria. Briefly, defatted milk samples were thawed at room temperature and individual vials were vortexed for uniform mixing. All standard milk samples supplied by the IAEA were reconstituted with 1 ml distilled water, vortexed and were used for assessing the presence of progesterone in unknown milk samples. The progesterone concentration was determined in duplicate samples. Anti-progesterone antibody coated tubes were loaded individually with 100µl of defatted milk and 1.0 ml of ratio-active iodine. Interval between calving to first ovulation were detected the rise of progesterone concentration to >3.0 nmol/L from immediate previous 10 days low value of >1 nmol/L around.
Analysis of the data
On the basis of progesterone concentration in milk, the cows initiating their post-partum ovarian activity was calculated. The values were expressed as mean ±SD. The data were analysed by using Statistical Packages for Social Sciences (SPSS) programme, version 10. One way analysis of variance (ANOVA) was done to compare body weight, BCS, milk yield at calving and at ovulation, interval between calving and ovulation among the groups.
RESULTS
Cows with higher BCS at calving (3.0±0.9) and at ovulation (2.7±0.9) required shorter (107.0±5.0 days) calving to ovulation and cows with lower BCS at calving (2.5±0.6) and at ovulation (1.9±0.3) required 234.1±66.6 days (p<0.05). Table 1 shows the effect of BCS at calving and at ovulation to the onset of postpartum ovarian cyclicity in cows.
The Heavier (286.6±89.0 kg) cows started their postpartum ovarian cyclicity earlier (107.0±5.0 days) than that of their lighter (219.2±0.3 kg) counterparts (234.1±66.6 days) (p<0.05). However, higher milk production (4.9±4.7 kg) and BCS (3.0±0.9) was recorded in cows with high body weight at calving. In addition, lower milk production and BCS (1.8±0.9 kg and 2.5±0.6; respectively) was recorded in cows with low body weight at ovulation (p< 0.05). Effects of body weight on the onset of postpartum ovarian cyclicity in cows are presented in Table1.
Higher milk yielding (6.3±3.5 litre/day) cows started their postpartum ovarian cyclicity earlier (77.4±6.5 days) compared to lower milk yielding (1.8±0.9 litre/day) cows that required (234.1±66.6) days (p<0.05). Higher body condition score and body weight at calving (3.0±0.9 and 283.9±34.7 kg) were recorded in high milk yielding cows. Similarly lower BCS and body weight (1.8±0.9 and 219.2±0.3 kg) at calving resulted in low milk production of cow (p<0.05). Effects of daily milk yield on onset of postpartum ovarian cyclicity in cows are presented in Table 1.
Progesterone concentrations in milk of individual cows sampled at 10 days intervals with BCS and milk production recorded at the time of milk sampling are presented in figures 1-5.
DISCUSSION
A wealth of information has been published concerning the effect of body condition score, body weight and milk yield on postpartum reproductive activity. The present study indicated that cows having >3.0 body condition score (1-5 scales) exhibited postpartum ovarian cyclicity earlier than those of cows having 1.0-<3.0 BCS at calving. The results were in agreement with findings of (Randel, 1990; Diag et al., 1991; Bolanos et al., 1996). Such cows loose rather much weight at postpartum (Heinonen et al., 1988) and often suffer from negative energy balance (Butler and Smith, 1989). The negative energy balance is associated with poor reproductive performance (Sasser et al., 1988). It has been observed that cows with negative energy balance fail to initiate pulsatile release of LH causing reduced ovarian function (Alam and Dobson, 1986). However, (Haresing, 1980) stated that poor health condition did not always have prolong postpartum open period. Alam and Dobson, 1986 suggested that best supplementation at prepartum will initiate earlier cyclicity in cows The cows in good body condition at parturition return to estrous earlier than cows in poor body condition (Richards et al 1986; and Darwash et al., 1996). Nutrition is probably the main factors involved in regulating GnRH secretion and hence LH pulse frequency (Randel, 1990).
Body weight at calving is an important determinant for the onset of postpartum ovarian cyclicity. The present study revealed that cows having >300 body weight showed post-partum ovarian cyclicity earlier than those having <300 body weight at calving. It has been postulated that energy balance and body weight played an important role in determining the postpartum interval to first ovulation and subsequent fertility (Britt, 1995; and Senatora et al., 1996). Doren et al. (1986) and Richards et al. (1986) demonstrated a positive correlation between the weight of cows at parturition and the time required for completion of uterine involution.
Pleasants and Barton, (1992) reported that the cows gaining weight following calving began their ovarian cyclicity earlier. Gain in body weight within 120 days of normal parturition resulted in earlier onset of oestrus. This may be due to earlier pituitary maturation. Conversely, the cows losing body weight following parturition showed impaired reproductive performance . The weight loss and decreased body fat at calving which ultimately reduced the reproductive efficiency was attributed to Dietary restriction during late pregnancy(Dziuk and Bellows, 1983) and both pre and postpartum dietary restriction ( Richards et al. 1986) . However, Rao and Venkatrmish (1993) did not find any correlation between the postpartum ovarian cyclicity and good body weight at calving.
ACKNOWLEDGEMENTS
The authors are grateful to Chief, SPCA, Noida for providing the samples and Dr. Amit Kumar Dinda, Additional Professor, Department of Pathology, AIIMS, New Delhi for providing the facilities for histopathological studies.
REFERENCES
-
Alam MGS, Dobson H. Postpartum
release of prostaglandin F2 alpha (PGF2 alpha) and the effect of
oestradiol benzoate on the concentrations of PGF2 alpha.
Bangladesh Vet J 1986; 20: 73-81.
-
Bolanos JM, Meneses A, Forsberg
M. Resumption of ovarian activity in zebu cows in the humid
tropics. Trop Anim Health Prod 1996; 28: 237-246.
-
Britt JH. Relationships between
postpartum nutrition, weight loss and fertility. Cattle
Practise1995; 3:79-83.
-
Butler WR, Smith RD.
Inter-relationship between energy balance and postpartum
reproductive function in dairy cattle. J Dairy Sci 1989;
72:767-783.
-
Claycomb RW, Delwiche MJ, Munro
CJ, Bondurant RH. Enzyme immunoassay for on-line sensing of milk
progesterone. Transactions of the ASAE 1996; 39: 729-734.
-
Coleman DA, Thayne WV, Dailey RA.
Factors affecting reproductive performance of dairy cows. J
Dairy Sci 1985; 58: 1793-1803.
-
Darwash A O, Lamming G E,
Wolliams J A. Estimation of genetic variation in the interval
from calving to post-partum ovulation of Dairy Cows. J Dairy Sci
1996; 80: 1227-11234.
-
Diag FMGN, Saturnino HM, Ruas
JRM, Norte AL, Oliveira HN. Effects of body condition, body
weight and the ratio of body weight to height on service period
in Nalore beef cows: Anais, IX Congresso Brasileiro de
Reproducao Animal, Belo-Horizonte, Brazil, 1991; 2: 288-291.
-
Doren PE, Long CR, Cartweight TC.
Factors affecting the relationship between calving interval of
cows and weaning weight of calves. J Anim Sci 1986; 62:
1194-1202.
-
Dziuk PJ, Bellows RW. Management
of Reproduction in beef cattle, sheep and pig. J Anim Sci 1983;
57: 355-360.
-
Fitzpatrick LA. Advances in the
under standing of post-partum anoestrus Bos indicus cows proc.
Final Research Co-ordinating meeting FAO/IAEA Co-ordinated
Research Programme, Bangkok, Thailand, 1-5 Feb. 1994; pp. 19-35.
-
Garcia M. On the reproductive
efficiency of pure and crossbred zebu cattle in the Amazon Basin
of Peru. Ph.D. Thesis. Swedish University of Agricultural
Science, Uppsala, Sweden. 1988.
-
Haresign W. Body condition milk
yield and reproduction of cattle In: Recent Advances in Animal
Nutrition, 1980. Haresign, W. and Lewis, D. Butterworth, London,
eds. 1980; pp. 107-122.
-
Haresign W, Foxcroft GR, Lamming
GE. Control of ovulation in farm animals. J Repord Fertility
1983; 69: 383-395.
-
Heinonen K, Ettala E, Alanko M.
Effect of postpartum live weight loss on reproductive functions
in dairy cows. Acta Vet Scand 1988; 29: 249-254.
-
Kamal MM. A review on cattle
reproduction in Bangladesh. International J Dairy Sci 2010; 5:
245-252.
-
Morrow DA. Current Therapy in
Theriogenology 2. (diagnosis, treatment and prevention of
reproductive diseases in small and large animals) 2nd ed. W.B.
Saunder Company Philadelphia, London, Toronto. 1986; pp.
437-442.
-
Nicholson MJ, Butterworth MH. A
Guide to Condition Scoring of Zebu Cattle. International
Livestock Centre for Africa. Addisababa, Ethiopia. 1986; pp 29
-
Opsomer G, Mijtem P, Coryn M,
Kruif ADE. Postpartum anoestrus in dairy cows. a review. Vet
Quarter 1996; 18: 68-75.
-
Pleasants AB, Barton RA. Effects
of different rates of live-weight change from 60 days before
calving to calving on the productivity of mature Angus breeding
cows. Newzealand J Agri Res 1992; 35: 199.
-
Randel RD. Nutrition and
postpartum rebreeding in cattle. J Anim Sci 1990; 68: 853-862.
-
Rao AVN, Venkatramaiah P. Effect
of body weight changes on the reproductive performance of
postpartum Ongole cows. Livestock-Adviser 1993; 18: 9: 10-12.
-
Richards MW, Spitzer JC, Warner
MB. Effect of varying levels of postpartum nutrition and body
condition at calving on subsequent reproductive performance in
beef cattle. J Anim Sci 1986; 62: 300.
-
Sasser RG, Williams RT, Bull RC,
Ruder CA, Falk DG. Postpartum reproductivity performance in
crude protein restricted beef cows: return to estrus and
conception, J Anim Sci 1988; 66: 3033-3039.
-
Senatora EM, Butler WR, Oltenacu
PA. Relationships between energy balance and post-partum ovarian
activity and fertility in first lactation dairy cows. J Anim Sci
1996; 62: 17-23.
-
Shamsuddin M, Bhuiyan MMU, Chanda
PK, Alam MGS, Abedin J. Fertility related factors at Artificial
Insemination in cattle in Bangladesh. Proc. BSVER Symposium on
Reproductive Health Management in Ruminants BAU, Mymensingh,
Publication No. 1997; 9: 21-33.
-
Shamsuddin M, Bhuiyan MMU, Chanda
PK, Alam MGS, Galloway D. Radioimmunoassay of milk progesterone
as a tool for fertility control in smallholder dairy farms. Trop
Anim Health Prod 2006; 38: 85-92.
-
Torres E. Postpartum adrenal
pituitary and ovarian functions in dairy cows. J Vet Med 1997;
44: 133-142.
TAbles
Table1. Effects of body condition score, body weight and milk yield on the calving to ovulation interval
|
Postpartum period (day)
|
Body weight (Kg)
|
BCS* at calving
|
BCS* at ovulation
|
Milk yield (litre)
|
Interval between calving to first ovulation (day)
|
|
10-60 (n=11)
|
283a ± 73.0
|
2.8 ± 0.6
|
2.7a ± 0.6
|
5.4a ± 3.6
|
34.7a ± 16.4
|
|
61-90 (n=10)
|
283.9a ± 34.7
|
2.7 ± 0.6
|
2.7a ± 0.6
|
6.3a ± 3.5
|
77.4b ± 6.5
|
|
91-120 (n=11)
|
286.6a ± 89.0
|
3.0 ± 0.9
|
2.7a ± 0.9
|
4.9a ± 4.7
|
107.0c ± 5.0
|
|
121-150 (n=9)
|
251.0ab± 68.0
|
2.6 ± 0.7
|
2.3ab ± 0.6
|
2.9ab ±1.7
|
135.9d ± 7.2
|
|
151-381 (n=9)
|
219.2b ± 0.3
|
2.5 ± 0.6
|
1.9b± 0.3
|
1.8b ± 0.9
|
234.1e ± 66.6
|
FIGURES
Fig. 1: Milk progesterone concentrations (nmol/L) of cow that had calving to first ovulation between Day 51 and Day 71 together with BCS (1-5 scale) and milk yield (kg/day).
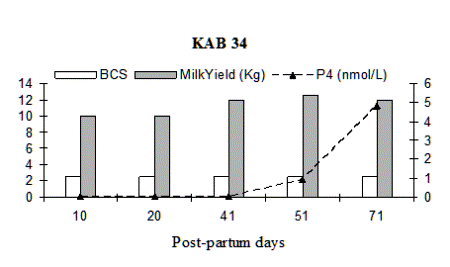
Fig. 2: Milk progesterone concentrations (nmol/L) of cow that had calving to first ovulation between Day 80 and Day 100 together with BCS (1-5 scale) and milk yield (kg/day).
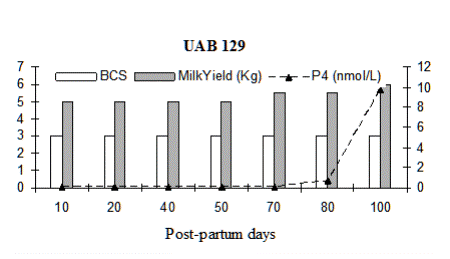
Fig. 3: Milk progesterone concentrations (nmol/L) of cow that had calving to first ovulation between Day 112 and Day 132 together with BCS (1-5 scale) and milk yield (kg/day)
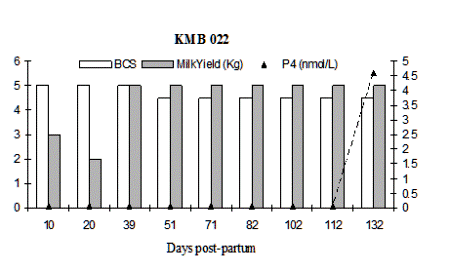
Fig. 4: Milk progesterone concentrations (nmol/L) of cow that had calving to first ovulation between Day 132 and Day 142 together with BCS (1-5 scale) and milk yield (kg/day)
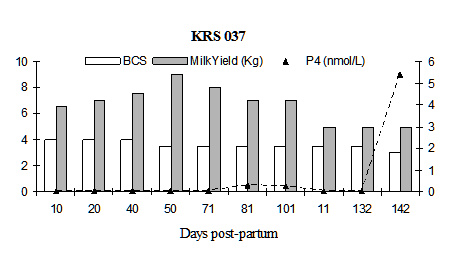
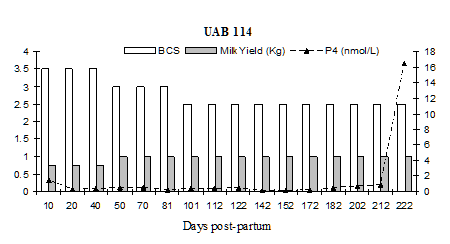
Fig. 5: Milk progesterone concentrations (nmol/L) of cow that had calving to first ovulation between Day 212 and Day 222 together with BCS (1-5 scale) and milk yield (kg/day)
|
 |







