|
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|
 |
|
||||||||||||||||||||||||||||||||||||||||||||
|
|
|
 |
|
|||||||||||||||||||||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||
|
2010, Vol. 5 No. 2, Article 60
Circadian Rhythm – A Review Dipak Banerjee*1and Anjan Dandapat2
1Ph.D. Scholar (Animal
Physiology)
*Corresponding Author; e-mail address: [email protected]
ABSTRACT The physiological processes of organisms are regulated by a circadian rhythm. The circadian rhythm is regulated by the wavelength, intensity, timing and duration of the light stimulus. Biological rhythms affect the sleep–wake cycle, migration behaviour in birds, seasonal fattening, hibernation and reproductive cycles in animals. The circadian rhythm is ubiquitous in nature. Circadian rhythms appear to be generated at the cellular level. Daily biological rhythms are endogenously controlled by self-contained circadian clocks. The period length is controlled by a circadian oscillator (clock). The timing of sleep and wakefulness under natural conditions is in synchrony with the circadian control of the sleep cycle and all other circadian-controlled rhythms. Adverse effects may ensue when the sleep-wake cycle is out of phase with the rhythms that are controlled by the circadian clock. Circadian rhythm is related to the light/dark cycle of the solar day but it also persists in constant conditions. The environmental cues entraining the circadian rhythm are called Zeitgebers or circadian synchronizers. Temporal restrictions of feeding (RF) can phase-shift behavioural and physiological circadian rhythms in mammals. Phase advances of circadian rhythms happens for instance, in the liver, kidney, heart, pancreas and some brain structures, uncoupling them from the control of the SCN, whose entrainment to light remains intact. KEY WORDS Circadian rhythms, molecular basis, circadian clock, light, feeding. INTRODUCTION
All the living organisms are exposed to the earth’s revolution around the sun with its cycle of day and night, of light and darkness and with the periodic changes in the length of the daily light and dark span along with the changes in seasons. Due to the rhythmicity of day and night most of the species exhibit daily changes in their behavior and/or physiology which generally arise from a timekeeping system within the organism. This timekeeping system is known as biological “clock” which allows the organisms to anticipate and prepare for the changes in the physical environment, thereby ensuring that the organism will “do the right thing” at the right time of the day. The biological clock provides internal temporal organization and ensures internal changes in coordination with one another (Vitaterna et al., 2001).
BIOLOGICAL RHYTHMS
Biological rhythms affect the sleep–wake cycle, migration behaviour in birds, seasonal fattening, hibernation and reproductive cycles in animals. In the 1950's Colin Pittendrigh and Jürgen Aschoff carried out research work on circadian rhythmicity in fruit flies and humans, respectively. They are considered as founder of chronobilogy. The area of sleep research, which also is subsumed under the field of chronobiology, began to develop independently, with the identification of various sleep stages by Nathaniel Kleitman around the same time (Dement, 2000).
CHARACTERISTIC FEATURES OF CIRCADIAN RHYTHMS
According to De Mairan’s observations the circadian rhythm is self-sustained in nature. Thus, almost all diurnal rhythms that occur under natural conditions continue to cycle under laboratory conditions devoid of any external time giving cues from the physical environment. Circadian rhythms that are expressed in the absence of any 24-hour signals from the external environment are called free running. This indicates that the rhythm is not synchronized by any cyclic change in the physical environment. A diurnal rhythm should not be called circadian until it has been shown to persist under constant environmental conditions and thereby can be distinguished from those rhythms that are simply a response to 24-hour environmental changes. However, almost all diurnal rhythms are found to be circadian.
MOLECULAR BASIS OF CIRCADIAN RHYTHMS Random mutation was carried out into the DNAs of the fruit fly, Drosophila melanogaster, and of the filamentous fungus Neurospora by using several mutagens. Then the resulting mutant organisms were screened for rhythm abnormalities. This mutagenesis approach led to the discovery of the first circadian clock mutants, which were called period (per) and frequency (frq, pronounced “freak”). The genes that carried the mutations in these organisms were cloned in the 1980s (Wager-Smith and Kay, 2000). However, researchers sought to isolate the equivalent genes in mammals (i.e., mammalian homologs'). Finally, in 1994, researchers began a similar mutagenesis screening approach in the mouse and described the first mouse circadian mutation, called Clock (King and Takahashi, 2000). In 1997 the gene affected by this mutation became the first mammalian circadian clock gene to be cloned (King and Takahashi, 2000). Recent advances in molecular biology and genetics led to the cloning of many mammalian ‘‘clock’’ genes and to the discovery of new, extracerebral sites containing circadian oscillators (Yamazaki et al., 2000). Hierarchical architecture of circadian rhythm from gene, to cell, to nerve nuclei, to brain, and to system is depicted in Fig. 5. CIRCADIAN CLOCK Daily biological rhythms are endogenously controlled by self-contained circadian clocks. The suprachiasmatic nuclei of the hypothalamus (SCN) are believed to be the anatomical locus of the circadian pacemaker (Silver and Moore, 1998). The period length is controlled by a circadian oscillator (clock) (Ikonomov et al., 1998). The biological timer can act as an alarm clock to initiate a physiological process of an organism at an appropriate phase of the daily environmental cycle. It can also help an organism prepare in anticipation of actual need. Another important function in some organisms is the accurate measurement of ongoing time throughout the daily cycle. The circadian clock can act like an instrument for estimating the day length or night length: thus, seasonal phenomena which respond to changing of day length can be regulated appropriately (Dunlap et al., 2004). This circadian oscillator, entrained by the light-day cycle via the retinohypothalamic tract, can impose circadian patterns on a wide array of physiological and behavioural processes (Cassone and Stephan, 2002). Physiological functions under the control of biological clock are given in Table 2. ANATOMICAL ORGANIZATION OF THE CIRCADIAN CLOCK
Studies of unicellular organisms depict the cellular nature of the system generating circadian rhythms. In higher organisms the circadian pacemaker is located in cells of specific structures of the organism. These structures are present in certain regions of the brain (i.e., the optic and cerebral lobes) in insects; the eyes in certain invertebrates and vertebrates; and the pineal gland in non mammalian vertebrates. In mammals, the circadian clock resides in two clusters of nerve cells called the suprachiasmatic nuclei (SCN), which are located at the anterior hypothalamus. The landmark discovery in the early 1970s demonstrated that the SCN is the site of primary regulation of circadian rhythmicity in mammals gave researchers a focal point for their research. By damaging (i.e., lesioning) the SCN in rats, researchers could disrupt and abolish endocrine and behavioral circadian rhythms (Klein et al., 1991). Furthermore, by transplanting the SCN from other animals into the animals with the lesioned SCN, researcher could restore some of the circadian rhythms. Finally, the SCN’s role as a master pacemaker regulating other rhythmic systems was revealed by similar studies in hamsters, which demonstrated that the restored rhythms exhibited the clock properties (i.e., the period, or phase, of the rhythm) of the donor rather than of the host (Ralph et al., 1990).
EFFECT OF SCN ON SLEEP-WAKE CYCLE
Although the effects of SCN lesions on numerous rhythms have been elucidated, their effects on sleep are less clear. Thus, SCN lesions clearly disrupt the consolidation and pattern of sleep in rats but have only minimal effects on the animals’ amount of sleep or sleep need (Mistlberger et al., 1987). Sleep is subject to two essentially independent control mechanisms: IMPORTANCE OF THE CIRCADIAN CLOCK
Nearly all physiological and behavioral functions in animals occur on a rhythmic basis, which in turn leads to dramatic diurnal rhythms in animal performance capabilities. A disturbed circadian rhythmicity in animals has been associated with a variety of mental and physical disorders and may negatively impact safety, performance, and productivity. Adverse effects of disrupted circadian rhythmicity may be linked to disturbances in the sleep-wake cycle. Some rhythmic processes are more affected by the circadian clock than by the sleep-wake state, whereas other rhythms are more dependent on the sleep-wake state.
CIRCADIAN RHYTHMS REGULATION BY LIGHT
Several experimental results show that light is the most important synchronizer of circadian rhythms. Light sets and resets the timing of the circadian timekeeping system, to ensure its proper functioning. Light exposure early in the morning resets the circadian system to adjust for its propensity to phase-delay, and light exposure in the evening is necessary to adjust for phase-advances in the master clock (Czeisler et al., 1990, Lewy et al., 1987).
CIRCADIAN RHYTHMS REGULATION BY FEEDING Feeding-entrained circadian system seems to be independent of the light-dark fluctuations of the solar day in different animals (Mistlberger, 1994; Stephan, 2002). Temporal restrictions of feeding (RF) can phase-shift behavioural and physiological circadian rhythms in mammals. It is postulated that changes in biological rhythms are caused by a food-entrainable oscillator (FEO), independent of the SCN (Mieda et al., 2006). In restricted feeding condition a (single period) scheduled at a fixed time of the day, mice (Mus musculus) adapt to this condition within a few days by feeding during the period of food availability and increasing food-seeking activity in the preceding hours (food anticipatory activity, FAA) (Hastings et al., 2003; Lowrey and Takahashi, 2004). Phase advances of circadian rhythms happens, for instance, in the liver, kidney, heart, pancreas and some brain structures, uncoupling them from the control of the SCN, whose entrainment to light remains intact (Damiola et al., 2000; Hara et al., 2001; Stokkan et al., 2001; Wakamatsu et al., 2001; Mendoza, 2006). It is postulated that feeding fasting signals may be involved in the entrainment of the peripheral circadian oscillators (Damiola et al., 2000; Stokkan et al., 2001). Existence of the FEO has not been unequivocally established. Some studies suggest that the dorsomedial hypothalamic nucleus (DMH) is a key structure for FEO expression (Gooley et al., 2006, Mieda et al., 2006). The results of few studies in rats with electrolytic DMH lesions do not, however, support this hypothesis. The circadian mechanism of FEO at the molecular level is not clear (Mendoza, 2006). The evidence supporting the existence of this feeding-entrained circadian system has been obtained only during restriction of feeding (RF); it is likely that if such a system exists it would also participate in the regulation of body rhythms in everyday conditions. REFERENCES
Aschoff J. Exogenous and endogenous components in
circadian rhythms.
Bartness TJ, Song CK, Demas GE. SCN efferents
to peripheral tissues: implications for biological rhythms. J
Biol Rhythms 2001; 16:196-204. Brainard GC, Podolin PL, Leivy SW, Rollag MD,
Cole C, Barker FM. Near-ultraviolet radiation suppresses pineal
melatonin content. Endocrinology 1986; 119:2201-2205. Brainard GC, Richardson BA, King TS, Matthews
SA, Reiter RJ. The suppression of pineal melatonin content and
N-acetyltransferase activity by different light irradiances in
the Syrian hamster: a dose-response relationship. Endocrinology
1983; 113:293-296. Buijs RM, Scheer FA, Kreier F, Yi C, Bos N,
Goncharuk VD, Kalsbeek A. Organization of circadian functions:
interaction with body. Prog Brain Res 2006; 153:341-360. Cardinali DP, Larin F, Wurtman RJ. Control of
the rat pineal gland by light spectra. Proc Nat Acad Sci
Cassone VM, Stephan FK. Central and
peripheral regulation of feeding and nutrition by the mammalian
circadian clock: Implications for nutrition during manned space
flight. Nutrition 2002; 18:814-819. Chesworth MJ, Cassone VM, Armstrong SM.
Effects of daily melatonin injections on activity rhythms of
rats in constant light. Am J Physiol 1987; 253:R101-R107.
Czeisler CA, Richardson GS, Zimmerman JC,
Moore-Ede MC, Weitzman ED.
Entrainment of human circadian rhythms by light-dark cycles: a
reassessment.
Photochem Photobiol 1980;
34:239-247. Damiola F, Le N, Preitner N, Kornmann B,
Fleury-Olela F, Schibler U. Restricted feeding uncouples
circadian oscillators in peripheral tissues from the central
pacemaker in the suprachiasmatic nucleus.
Genes Dev
2000;
14:2950-2961. Dement WC. History of sleep physiology and
medicine. In: Kryer MH, Roth T, Dement WC, eds.
Principles and Practice
of Sleep Medicine. 3rd Edition.
Deprés-Brummer P, Lévi F, Metzger G, Touitou
Y. Light-induced suppression of the rat circadian system. Am J
Physiol 1995; 37:R1111-R1116. Dunlap JC,
Loros JJ, DeCoursey PJ. Fundamental properties of circadian
rhythms. In: Chronobiology – Biological Timekeeping, Sinauer
Associates, Inc. Publishers,
Eastman C, Rechtschaffen A. Circadian
temperature and wake rhythms of rats exposed to prolonged
continuous illumination. Physiol Behav 1983; 31:417-427.
Franken P, Lopez-molina L, Marcacci L,
Schibler U, Tafti M. The transcription factor DBP affects
circadian sleep consolidation and rhythmic EEG activity.
Journal of Neuroscience 2000; 20(2):617–625.
Gooley JJ, Schomer A, Saper CB. The
dorsomedial hypothalamic nucleus is critical for the expression
of food-entrainable circadian rhythms.
Nature
Neurosci 2006; 9:398-407.
Hara R, Wan
K, Wakamatsu H, Aida R, Moriya T, Akiyama M, Shibata S.
Restricted feeding entrains liver clock without patricipation of
the supraciasmatic nucleus. Genes Cells
2001;
6:269-278.
Haus E, Halberg F. Circannual rhythm in level
and timing of serum corticosterone in standardized inbred mature
C-mice. Environ Res 1970; 3:81-106. Homna K, Hiroshige T. Endogenous ultradian
rhythms in rats exposed to prolonged continuous light. Am J
Physiol 1978; 235:R250-R256.
Ikonomov OG, Stoynev AG, Shisheva AC. Integrative coordination
of circadian mammalian diversity: Neuronal networks and
peripheral clocks. Progress in Neurobiology 1998; 54:87–97. King DP, Takahashi JS. Molecular genetics of
circadian rhythms in mammals.
Annual Review of Neuroscience 2000; 23:713–742.
la Fleur SE. Daily rhythms in glucose
metabolism: suprachiasmatic nucleus output to peripheral tissue.
J Neuroendocrinol 2003; 15:315-322. Lewy AJ, Sack RL, Singer CM.
Immediate and delayed
effects of bright light on human melatonin production: shifting
"dawn" and "dusk" shifts the dim light melatonin onset (DLMO).
Horm Metab Res 1987; 19:437-440.
Meijer JH, Rietveld WJ. Neurophysiology of the suprachiasmatic
circadian pacemaker in rodents. Physiol Rev 1989; 69:671-707. Mendoza J. Circadian clocks: setting time by
food. J Neuroendocrinol 2006; 19:127-137. Mieda M,
Williams SC,
Mistlberger RE. Circadian food-anticipatory activity: formal
models and physiological mechanisms. Neurosci Biobehav Rev 1994;
18:171-195. Mistlberger RE, Skene DJ.
Nonphotic entrainment in
humans. J Biol
Rhythms 2005; 20:339-352. Mistlberger RE, Bergmann BM, Rechtschaffen A.
Relationships among wake episode lengths, contiguous sleep
episode lengths, and electroencephalographic delta waves in rats
with suprachiasmatic nuclei lesions.
Sleep 1987;
10(1):12–24.
Nicolau GY, Lakatua D, Sackett-Lundeen L,
Haus E. Circadian and circannual rhythms of hormonal variables
in clinically healthy elderly men and women (abstr).
Chronobiologia 1983; 10:144. Paranjpe DA, Sharma
K. Evolution of
temporal order in living organisms.
J Circadian Rhythms 2005;
3:7. Piccione G, Caola G.
Biological
Rhythm in Livestock.
J Vet Sci
2002; 3(3):145-157. Pittendrigh CS. Circadian rhythms and the
circadian organization of living systems.
Ralph MR, Foster RG, Davis FC, Menaker M.
Transplanted suprachiasmatic nucleus determines circadian
period. Science
1990; 247:975–978.
Redman J, Armstrong S, Ng KT. Free-running
activity rhythms in the rat: entrainment by melatonin. Science
1983; 219:1089-1091. Refinetti R. Biological Rhythms. In:
Circadian Physiology. CRC Press,
Rusak B, Zucker I. Neural regulation of
circadian rhythms. Physiol Rev 1979; 59:449-526. Silver R, Moore RY. The suprachiasmatic
nucleus and circadian function: An introduction. Chronobiology
International 1998; 15:7-10. Stephan
FK. The “other” circadian system: food as a Zeitgeber. J Biol
Rhythms 2002; 17:284-292. Stokkan KA, Yamazaki S, Tei H, Sakaki Y,
Menaker M. Entrainment of the circadian clock in the liver by
feeding. Science 2001; 291:490-493. Takahashi JS, DeCoursey PJ, Bauman L, Menaker
M. Spectral sensitivity of a novel photoreceptive system
mediating entrainment of mammalian circadian rhythms. Nature
1984; 308(5955):186-188. Thomas EMV, Armstrong SM. Melatonin
administration entrains female rat activity rhythms in constant
darkness but not in constant light. Am J Physiol 1988;
255:R237-R242. Vitaterna
Martha Hotz, Takahashi Joseph S, Turek Fred W. Overview of
Circadian Rhythms. Alcohol Research & Health 2001; 25(2):85-93. Wager-smith K, Kay SA. Circadian rhythm
genetics: From flies to mice to humans.
Nature Genetics 2000; 26:23–27.
Wakamatsu H, Yoishinobu Y, Aida R, Moriya T, Akiyama M, Shibata
S. Restricted-feeding-induced
anticipatory activity rhythm in associated with a phase-shift of
the expression in mPer1 and mPer2 mRNA in the cerebral cortex
and hippocampus but not in the suprachiasmatic nucleus of mice.
Eur J Neurosci 2001; 13:1190-1196.
Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block
GD, Sakaki Y, Menaker M, Tei H. Resetting central and
peripheral circadian oscillators in transgenic rats. Science
2000;
288:682-685.
Figures
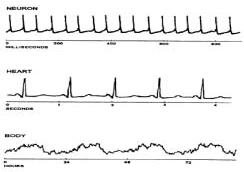
Fig. 1: Graphical representation of biological
rhythms (Source: Refinetti, 2000) Parameters of
circadian rhythm
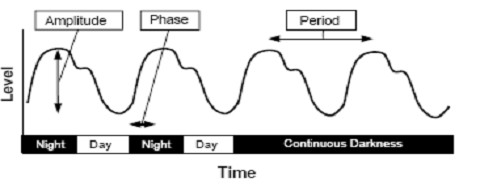
Fig. 2: A representative circadian rhythm is depicted in which the
level of a particular measure varies according to time. The
difference in the level between peak and trough values is the
amplitude of the rhythm. The timing of a reference point in the
cycle relative to a fixed event is the phase. The time interval
between phase reference points (e.g., two peaks) is called the
period. The rhythm shown persists even in continuous darkness.
(Source: Vitaterna
et al.,
2001) Resetting the
circadian rhythm
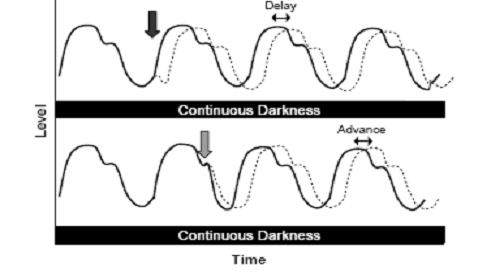
Fig. 3: In the case of a phase delay, the peak levels are reached
later than they would be had the rhythm not been shifted. In
the case of a phase advance, the peak levels are reached earlier
than they would be had the rhythm not been shifted. The black line
shows how cycling would appear if the rhythm remained unchanged.
(Source: Vitaterna
et al.,
2001)
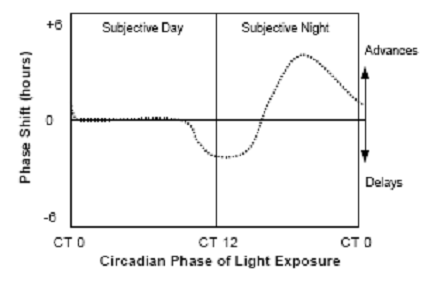
Fig. 4: Virtually all species show similar phase-dependent-resetting
responses to light, which can be expressed as a phase-response
curve. Exposure to light during the early part of the animal’s night
causes a phase
delay, whereas exposure to light in the latter part of the animal’s
night causes a phase advance. Light exposure during the animal’s
usual daytime period produces little or no phase shift. (Source:
Vitaterna et al., 2001)
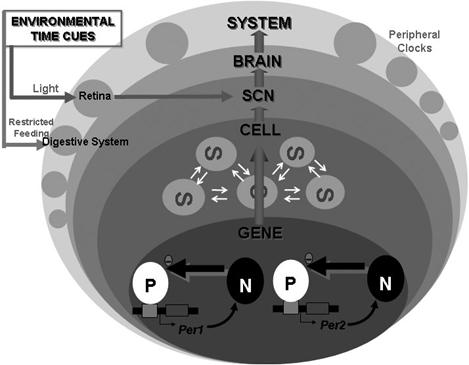
Fig.
5: ‘GENE’ depicts rhythmic transcription of mPer1 and mPer2.
‘CELL’ represents neuronal electrical activities of single SCN
neuron. ‘SCN’ indicates the sum of the local neuronal and glial
circuits. ‘BRAIN’ symbolizes functions produced by neuronal
circuits in the brain such as sleep and recognition. ‘SYSTEM’
symbolizes behavior, peripheral neuronal activities and hormonal
secretion. ‘P’ and ‘N’ at gene level represent positive and
negative elements respectively. Positive factors stimulate the
transcription of clock genes, and their translational products
negatively regulate the transcription of their own gene. At the
SCN, cell clocks interact with each other, and harmonize to make
a strong rhythm in the SCN as a whole. At the system level, many
of the peripheral organs have their own ‘peripheral clock’.
Environmental time cues enter into this
circadian system site-dependently. The master clock in the SCN
receives light information via the retina, and the presumed
peripheral clocks in the digestive system, such as that in the
liver, receive feeding information. (Source: Okamura, 2003)
Tables Table 1: Frequency ranger in biological rhythms
(Source: Piccione and Caola, 2002)
Table
2: Physiological functions under the control of biological clock
(Source: Piccione et al.,
2005)
|
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||
|
Copyright © Vet Scan 2005- All Right Reserved with
VetScan |
Home | e-Learning |Resources | Alumni | Forum | Picture blog | Disclaimer |
|
||||||||||||||||||||||||||||||||||||||||||||
|
powered by eMedia Services |
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||||||||||||||||||||