|

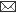
2010, Vol. 5 No. 2, Article 61
Stress Versus Reproduction in Animals
Z. A. Pampori*1, R. Huozha1, K. A. Shah2,
S. A. Andrabi2 and Tauseef Amin1
1Animal Physiology Division
National Dairy Research Institute,
Karnal (Haryana),
India-132001
2Department of Animal Husbandry
Kashmir
(J&K)
*Corresponding Author;
e-mail address: [email protected]
KEY
WORDS
Stress, reproduction, sympathetic nervous system, HPA
axis.
INTRODUCTION
Stress may be defined as a real or interpreted threat to physiological or psychological integrity that results in physiological and/or behavioral responses. (McEwen, 2000), or a state of disharmony, or threatened homeostasis. Stress challenges homeostasis, but does not disrupt it in an adapting organism.
(Rivier and Rivest, 1999; Chrousos and Gold, 1992).
Stressors may be categorised as, Physical - temperature; Chemical - toxicants; Nutritional - lack of food; Psychological - thought, feelings; Psychosocial - social interactions and Immunological – disease agents.
Whether a stimulus is an actual threat is not important, it is only the perception of a threat that is critical. Once CNS perceives a threat, it develops behavioral, autonomic, endocrine or immune response to maintain homeostasis. In case animal is unable to withstand stress, the consequences will be abnormal biological functions and development of pathologies. Behavioral response is most early and economical response, but not appropriate for all stressors. Autonomic system affects a diverse number of biological systems. Its short duration might argue its impact on animal’s long term welfare.
The endocrine system has broad, long lasting effects virtually on all biological functions whereas immune responses boost up the defense mechanism of the animal.
The type of biological defense an animal utilizes is not important but the resulting change in biological function determines if there is a threat to the animal welfare. Change in biological functions
results in shift of biological resources away from the biological activities occurring before stress. Several biological events (particularly reproductive)
that rely on critical timing for success get hampered if disturbed due
to stress. Stress turns to distress when body reserves are depleted and
many biological functions are compromised, yet basal functions remain maintained. Distress, however, is overcome during recovery when body reserves are restored and biological functions operate normally. Stress acts at multiple neuro-endocrine and organ levels and at all stages of life irrespective of sex.
Stress can affect every organ system in the body, but the principal mediators of the response to stress are the sympathetic nervous system (catecholamines) and the hypothalamo-pituitary-adrenal axis (cortisol,
adrenocorticotropic hormone & corticotropin releasing factor) (McEwen, 2005). Number of endocrine, behavioral, autonomic and immune responses are measured but none has proved a litmus test for stress.
However, under controlled experimental conditions, cortisol can be a reliable indicator of stress.
Most relied markers of stress
A. Clinical markers
Elevated plasma levels of ACTH (adrenocorticotropic hormone)
Elevated plasma levels of Cortisol
Elevated plasma levels of Adrenalines/ catecholamines
Neutrophilia
Lymphopenia
Hyperglycemia
B. Immunological markers
Reduced lymphocytic mitotic activity
Reduced secretion of IL-2
Reduced size of lymph nodes and thymus
C. Heat shock proteins (HSP)
Recently HSPs discovery has added one more reliable marker of stress. Up-regulation of genes involved in response to stress include those of HSP, that act as chaperones and promote greater tolerance/resistance to stress (thermic and others).
STRESS CONTROL SYSTEM
In the most simplistic model, the hypothalamic sympathetic (HS) and the hypothalamic-pituitary-adrenal (HPA) systems provide brain and periphery control of stress responses. The HS system, beginning with neurons (the dorsal parvocellular and ventromedial parvocellular) in the paraventricular
nucleus (PVN), is responsible for the catecholamine system. It is
activated when animal experiences stress, causing release of catecholamine from both the brain and the adrenal medulla (Valentino and Van Bockstaele,
2005). The PVN has both afferent neurons entering the nuclei and
efferent neurons departing the PVN. The afferent neurons arrive from
two directions; from the brain’s cortex down to the PVN or from the
periphery up to the PVN. Purely psychological stressors arrive in
the PVN from higher cortical brain centers. Peripheral neurons that
sense temperature, touch, and pain arrive at the PVN from the
periphery. Sensory neurons also make their way to the cortex where
the signal can be relayed to the PVN from the stress that may have
an emotional (higher) component. Restraint and most acute stressors activate only the medial parvocellular-PVN, the site of Corticotropin Releasing Factor
(CRF) secretion.
Reasons for elevated glucocorticoids essential for resisting stress, remains for most part unknown. Hypophysectomized or adrenalectomized animals treated with maintenance dose of glucocorticoids die when exposed to noxious stimuli. Epinephrine/catcholamines are first to increase
,within minutes, during stress with peak values reaching in less than 30 minutes but have very short half-lives
and are metabolized and cleared rapidly. Cortisol on other hand takes several minutes and reach peak values within 1 to 3 hours. It lasts longer in circulation, results in the induction of new proteins and exhibits long-term effects.
STRESS MODULATES HYPOTHALOMO-PITUITARY AXIS
Reproduction is an energy consuming activity and has to infer overall health of the animal, including situations in which survival is in question. Hence, of all the body systems, the reproductive is most expendable when homeostasis is in jeopardy and survival of the individual most often comes before reproduction. In situations where adaptation to stress is surrendered, the animal faces pre-patho-physiological state which may lead to pathologies like subfertility and reproductive disorders (impaired oocyte quality; lowered pregnancy rates); mastitis; disturbed liver and immune function; infections; mortality and/or behavioural disturbances.
Stressors affect reproductive function via actions at the hypothalamus as well as
by impairing pituitary LH release induced by gonadotrophic releasing hormone (GnRH). The most pronounced effect of stress on reproduction is through modulation of LH secretion. Various studies have shown that administration of natural or synthetic glucocorticoids can inhibit the secretion of the gonadotrophins in sheep (Juniewicz et al., 1987), pigs (Turner et al., 1999 a & b), cattle (Thibier and Rolland, 1976), rhesus monkeys (Dubey and Plant, 1985) and humans (Saketos et al., 1993). Cortisol
inhibits LH pulse amplitude, however, LH pulse frequency was unaffected. Cortisol suppresses pulsatile LH secretion by inhibiting pituitary responsiveness to GnRH rather than by suppressing hypothalamic GnRH release (Breen and Fred, 2004).
There are anatomical connections between CRF axons and GnRH neuronal dendrites. CRF neuronal cells have been found in median pre optic area that significantly decreases GnRH. In vivo and in vitro evidence suggests direct action of glucocorticoids upon gonadotrophs of domestic animals to reduce gonadotropins, especially LH. Evidence suggests that the glucocorticoids may not be acting alone and that other hormones of the adrenal axis (such as CRH and ACTH) may play a role in the gonadotropin release and secretion. The primary action appears to block the ability of GnRH to stimulate the secretion of LH (Moberg and Mench, 2000). The ability of glucocorticoids to
directly inhibit GnRH release suggests that these stress-responsive hormones act also at the hypothalamic level to suppress the reproductive function. The early suppression of LH secretion by stressors is due to immediate activation of the sympathetic nervous system which in turn exerts complex stimulatory and inhibitory effects on GnRH release.
In dairy heifers, application of an acute stress treatment (electric shock, confinement, restraint and rotation) twice a day during the follicular phase of the oestrous cycle prevents the pre-ovulatory LH surge. Nutritional and metabolic stress around parturition in the high yielding dairy cows terminates into sub-fertility and reproduction disorders while transportation stress in dairy cattle reduces the amount of LH secreted in response to exogenous GnRH stimulation (Dobson and Smith, 1995). In sheep, restraint and confinement elevate the concentrations of plasma cortisol and at the same time decrease the pituitary’s response to exogenous GnRH (Tilbrook et al, 2002). Recent preliminary evidence in ovariectomized ewes demonstrates increased cortisol secretion is essential for disruption of pulsatile luteinizing hormone secretion in response to a psychosocial stress (Breen et al., 2006).
Leptin and adiponectin provide feedback to hypothalamus for GnRH release. Feed restrictions or malnutrition serves as stress and reduces leptin levels that alter reproductive functions. Leptin hormone may serve signal to the brain on the critical amount of fat stores that are necessary for GnRH secretion and activation of the hypothalamic-pituitary-gonadal axis. Falling leptin levels in response to starvation result in decreased estradiol levels and amenorrhea in subjects with anorexia nervosa or strenuously exercising athletes (Mantzoros et al., 1997). Conditions, in which nutritional status is suboptimal, such as eating disorders, exercise-induced amenorrhea, and functional hypothalamic amenorrhea, are associated with low serum leptin levels (Basdevant and Ciangura, 2007). Nutrient restriction during early pregnancy, when demands are minimal can have long term effects. GnRH pulse is highly sensitive to conditions like weight loss, decreased energy availability, altered body fat ratio (Beam and Butler, 1997). Rams had a greater cortisol response to insulin-induced hypoglycaemia than ewes while ewes showed a greater cortisol response to isolation/restraint stress than rams (Turner et al., 2002).
Extremes in climate alter energy transfer with deleterious effects on reproduction, possibly through cortisol mediation and increased caloric need, most obvious is summer sterility in rams and buffaloes or calf scrotal frostbite.
PLEIOTROPIC EFFECTS OF STRESS
There are multiple pathways of the many effects of stress at multiple stages in the reproductive process. Female effects may be more crucial in the sense that prevention of the LH surge, being such a timed event, is more detrimental than in males, at least where acute stress is involved.
a. Mating / Copulatory behavior: Depression, anxiety and chronic stress may interfere with central and peripheral pathways of the sexual response (decreased sex drive). Zoo animals’ exhibit decreased reproductive capacity associated with captivity. Heat stress dampens estrus behavior in cattle, estrus intensity and length decrease. Heat stress damages ovarian follicles and causes a decrease in estradiol synthesis. This decrease in estradiol synthesis could influence expression of estrus, ovulation, and the corpus luteum (Wilson et al., 1998a & b). Nebel et al. (1997) demonstrated that dairy cows in the summer had approximately one-half the number of mounts per estrus compared to dairy cows in the winter.
b. Gamete maturation: Stress results in disturbance of spermatogenesis, decreased sperm fertility parameters (summer sterility)
and disturbance of folliculogenesis. Stress may inhibit gonadotropic responsiveness in granulosa cells. Catecholamine interfere with transport of gametes and decreases blood flow (Breen et al., 2006).
There are various reports that indicate reduced oocyte quality, reduced pregnancy rates from AI or natural service, reduced developmental ability of embryos during periods of hot weather. Putney et al. (1989b) demonstrated that embryonic development was impaired in heifers subjected to heat stress for 10 hours after the onset of estrus. Ealy et al. (1993) found that heat stress on day 1 after breeding decreased subsequent embryonic development.
c. Pregnancy: Early embryonic loss in livestock is common due to stress. Increased incidence of spontaneous abortion, preterm delivery and low birth weight
may also result from it. Several studies in mammals have shown that maternal prenatal stress may induce over-activity and/or dysregulation of the HPA-system in the offspring (Mulder et al., 2002). Cattle, sheep in heat stress exhibit reduced uterine and umbilical blood flows, resulting reduced fetal oxygen, nutrients and fetal size (Reynolds et al., 1997).
d. Post-partum behavior: Stress during gestation alters postpartum maternal care and the development of offspring. Stress can directly alter maternal care through neuro-endocrine systems that normally regulate this behavior (Champagne and Meaney, 2006).
e. Behavior of offspring: In animal studies, prenatal maternal stress has been shown to affect postnatal physical outcome, and the development, behaviour, and stress responses of the offspring (Huizink et al., 2004a). Prenatal stress induces developmental and behavioral disorders. Female progeny are reported to become bad mothers (Mulder et al., 2002). Stress of weaning produces weaning shock predominantly in kids.
STRESS MANAGEMENT
Stress can be reduced by improving managerial practices and providing optimal inputs.
Diet - Should be balanced, complete, timely and as per the need in different physiological states.
Housing - Shelter management is critical in climate extremes, taking good care of temperature and ventilation.
Overcrowding/ isolation - Psycho-social needs of the animal are to be
addressed. Isolation and overcrowding should be avoided.
Transportation - restraint/captivity - Transportation
stress should be minimized and avoided during breeding periods.
Animals in captivity should be provided with conditions simulating
as far as possible, its natural habitat.
Use of anti-stress compounds - like antioxidants during oxidative stress.
REFERENCES
Beam, S. W and Butler, W. R
(1997). Energy balance and ovarian follicle development prior
to the first ovulation postpartum in dairy cows receiving three
levels of dietary fat. Biology of Reproduction 56: 133–142.
Basdevant A, Ciangura C
(2007). Leptin: from gene to energy balance. Exp. Clin.
Endocrinol
Diabetes. 115: 423-7.
Breen, M. K and Fred, J. K
(2004). Does cortisol inhibit pulsatile luteinizing hormone
secretion at the hypothalamic or pituitary level? Endocrinology
145: 692–698.
Breen, K. M; Oakley, A. E;
Pytiak, A.V; Tilbrook, A.J; Wagenmaker, E. R and Fred, J. K
(2006). Does cortisol acting via the Type II Glucocorticoid
Receptor mediate suppression of pulsatile luteinizing hormone in
response to psychosocial stress? Endocrinology 148: 1882–1890.
Champagne, F. A and Meaney, M.
J (2006). Stress during gestation alters postpartum maternal
care and the development of the offspring in a rodent model.
Biological Psychiatry.59: 1227-1235
Chrousos, G.P and Gold, P.W
(1992). The concept of stress and stress system disorders. J.
Am. Med. Ass. 267: 1244-1252.
Dobson, H and Smith, R. F
(1995). Stress and reproduction in farm animals. Journal of
reproduction and fertility. Supplement 49: 451-61.
Dubey, A. K and Plant, T. M
(1985). A suppression of gonadotropin secretion by cortisol in
castrated male Rhesus monkeys (Macaca mulatta) mediated by the
interruption of hypothalamic gonadotropin-releasing hormone
release Biology of Reproduction. 33: 423–431.
Ealy, A.D; Drost, M and
Hansen, P.J (1993). Developmental changes in embryonic
resistance to adverse effects of maternal heat stress in cows.
J. Dairy Sci. 76: 2899-2905.
Huizink, A.C., Mulder, E.J.H.,
Buitelaar, J.K., 2004a. Prenatal stress and risk for
psychopathology. Specific effects or induction of general
susceptibility. Psychol. Bull. 130: 115–142.
Juniewicz, P. E; Johnson, B.H
and Bolt, D.J (1987). Effect of adrenal steroids on testosterone
and luteinizing hormone secretion in the ram. Journal of
Andrology. 8: 190–196.
Mantzoros, C; Flier J.S; Lesem,
M. D; Brewerton, T. D and Jimerson, D.C (1997). Cerebrospinal
fluid leptin in anorexia nervosa: correlation with nutritional
status and potential role in resistance to weight gain. J Clin
Endocrinol Metab 82: 1845-1851.
McEwen, B (2000). Stress,
definition and concepts. In Encyclopedia of Stress. G. Fink, ed.
Academic Press. San Diego, CA. 3: 508-509.
McEwen, B. S (2005). Stressed
or stressed out: what is the difference? J. Psychiatry Neurosci.
30: 315–318.
Moberg, G.P and Mench, J.A
(2000).The biology of animal stress. CAB international.
Mulder, E.J.H; Robles de
Medina, P.G; Huizink, A.C; Van den Bergh, B.R.H; Buitelaar, J.K;
Visser, G.H.A ( 2002). Prenatal maternal stress: effects on
pregnancy and the (unborn) child. Early Hum. Dev. 70: 3–14
Nebel, R.L; Jobst, S.M;
Dransfield, M.B.G; Pandolfi, S.M and Bailey, T.L (1997). Use of
radio frequency data communication system, HeatWatch, to
describe behavioral estrus in dairy cattle. J. Dairy Sci. 80:
179.
Putney, D.J; Mullins, S;
Thatcher, W.W; Drost, M and Gross, T.S (1989b). Embryonic
development in superovulated dairy cattle exposed to elevated
ambient temperatures between the onset of estrus and
insemination. Anim. Reprod. Sci. 19: 37-51.
Reynolds, T. S; Stevenson, K.
R and Wathes, D. C (1997). Pregnancy specific alterations in the
expression of the insulin-like growth factor system during early
placental development in the ewe. Endocrinology. 138: 886–897.
Rivier, C. and Rivest, S
(1991).Effect of stress on the activity of the hypothalamic-
pituitary-gonadal axis:peripheral and central mechanisms Biology
of Reproduction. 45: 523-532.
Saketos, M; Sharma, N and
Santoro, N. F (1993). Suppression of the hypothalamicpituitary-
ovarian axis in normal women by glucocorticoids. Biol Reprod 49:
1270–1276.
Selye, H (1936). A syndrome
produced by diverse nocuous agents. Nature. 138: 32.
Thibier, M and Rolland, O
(1976). The effect of dexamethasone (DXM) on circulating
testosterone (T) and luteinizing hormone (LH) in young
postpubertal bulls. Theriogenology. 5: 53–60.
Tilbrook, A. J., Turner, A. I.
and Clarke,I. J (2002). Effects of stress on reproduction in
non- rodent mammals: the role of glucocorticoids and sex
differences. Reviews of Reproduction 5: 105–113.
Turner, A. I; Hemsworth, P.H;
Canny, B.J and Tilbrook, A. J (1999a). Inhibition of the
secretion of LH by sustained but not repeated acute elevation of
cortisol in the absence but not the presence of oestradiol.
Journal of Endocrinology. 163: 477–486.
Turner, A. I; Hemsworth, P. H;
Canny, B. J and Tilbrook, A. J (1999b). Sustained but not
repeated acute elevation of cortisol impaired the LH surge,
estrus and ovulation in gilts Biology of Reproduction. 61:
614–620.
Turner, A. I; Canny, B. J;
Hobbs, R. J; Bond, J. D; Clarke, I. J and Tilbrook, A. J (2002).
Influence of sex and gonadal status of sheep on cortisol
secretion in response to ACTH and on cortisol and LH secretion
in response to stress: importance of different stressors.
Journal of Endocrinology. 173: 113–122.
Valentino, R. J and Van
Bockstaele, E. J. (2005). Functional interactions between stress
neuromediators and the locus coeruleus-norepinephrine system.
Handbook of stress and the brain. 15: 465-486.
Wilson, S.J; Kirby, C.J;
Koenigsfeld, A.T; Keisler, D. H and Lucy, M.C (1998a). Effects
of controlled heat stress on ovarian function of dairy cattle..
J. Dairy Sci. 81: 2132-2138.
Wilson, S. J; Marion, R.S;
Spain, J.N; Spiers, D.E; Keisler, D.H and Lucy, M.C (1998b).
Effects of controlled heat stress on ovarian function of dairy
cattle. J. Dairy Sci. 81, 2124- 2131.
Figures
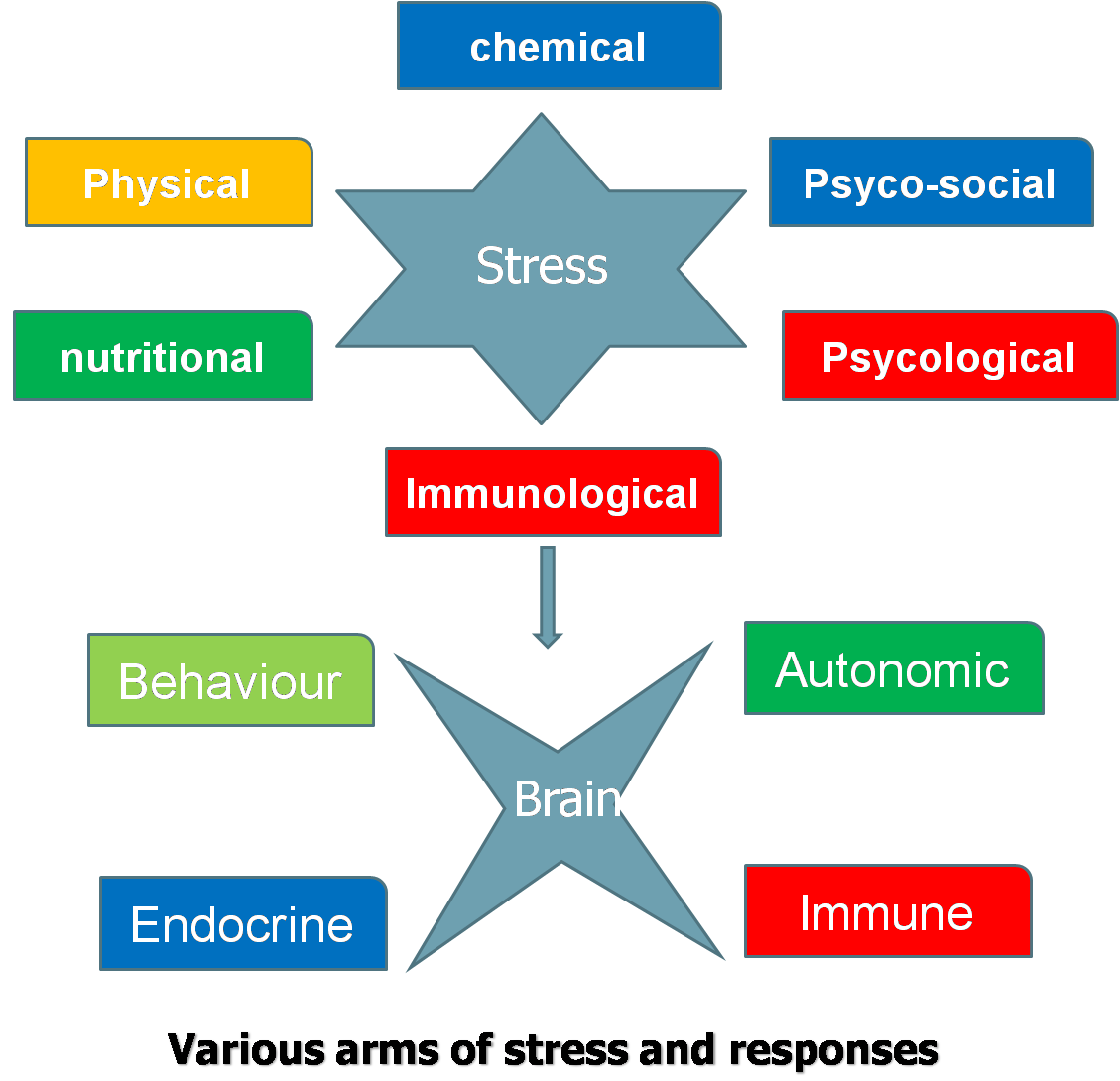
Fig. 1
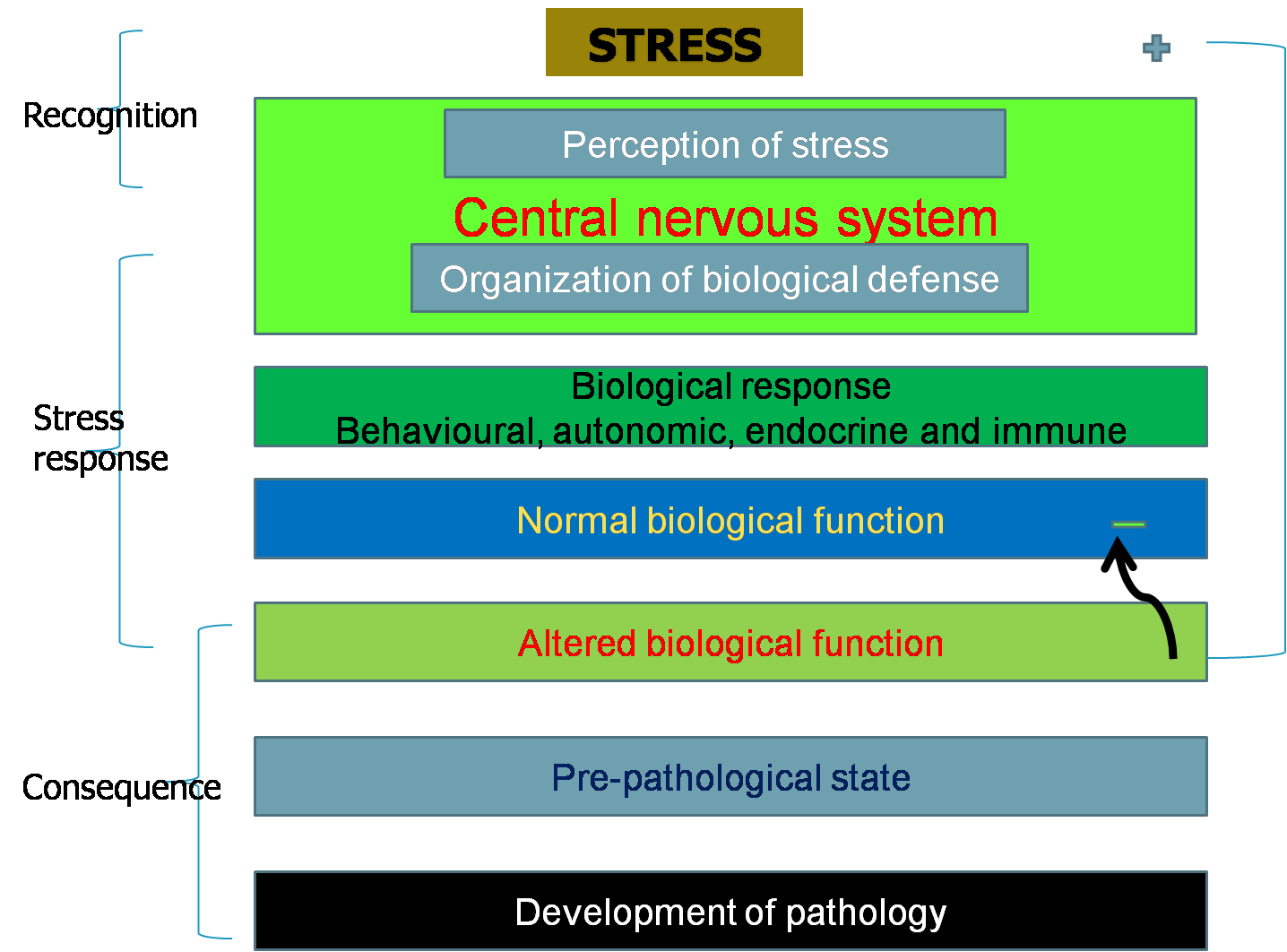
Fig. 2
Fig. 3.
|
 |





